Measles in Children- What Parents Need To Know
Measles is a highly contagious viral infection that primarily affects children. It spreads through coughing, sneezing, or direct contact with an infected person. The virus can remain in the air for up to two hours after an infected person leaves the area.
Concern for measles in children is because of recent measles outbreaks in children that ishappening in the western countries -,
West Texas (2025): 48+ cases, among unvaccinated children.
Ohio (2024): Outbreak in schools due to low vaccine rates.
Europe and UK (2024–2025): Increasing cases among unvaccinated toddlers.
Symptoms of measles in children: Symptoms usually appear 7–14 days after exposure and progress in stages:
Early symptoms (Days 1–4):
- High fever (can reach 104°F or higher)
- Runny nose
- Dry cough
- Red, watery eyes (conjunctivitis)
- Fatigue & loss of appetite
Koplik’s spots (Days 2–3):
- Tiny white spots with bluish-white centers appear inside the mouth on the inner cheeks.
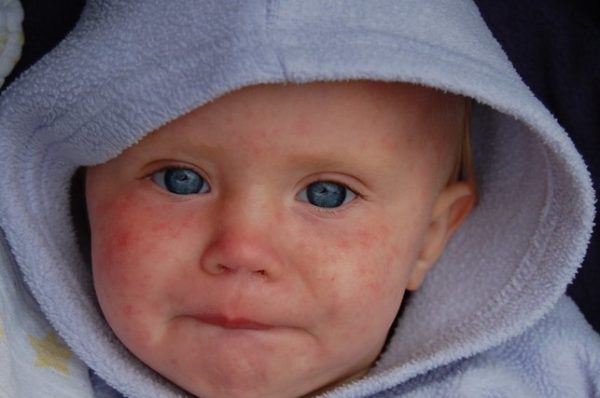
Measles rash (Days 3–5):
- Begins as flat red spots on the face and hairline.
- Spreads downward to the neck, trunk, arms, legs, and feet.
- May merge into large patches and last 5–7 days before fading.
What are some complications can parents expect? While most children recover, measles can cause serious complications, especially in infants, malnourished children, and those with weakened immune systems.
Common complications:
- Ear infections -which can lead to hearing loss.
- Diarrhea -causing dehydration.
Severe complications:
- Pneumonia -most common cause of death in measles cases
- Encephalitis -brain swelling, leading to seizures or disability.
- Subacute sclerosing panencephalitis (SSPE) – A rare but fatal brain disorder that can develop years after measles infection.
Prevention: Measles vaccination (MMR Vaccine):
The best way to prevent measles is vaccination with the MMR (Measles-Mumps-Rubella) vaccine.
MMR vaccine schedule for children:
First dose: 12–15 months old
Second dose: 4–6 years old
If a child misses a dose, they should get vaccinated as soon as possible.
How effective is the MMR vaccine?
- 1 dose = 93% protection against measles
- 2 doses = 97% protections for life
Other preventive measures:
- Handwashing: Encourage frequent handwashing with soap.
- Avoiding contact: Keep infected children isolated for at least 4 days after a rash appears.
- Post exposure prevention: If an unvaccinated child is exposed, they may still get the MMR vaccine within 72 hours or receive immune globulin (IG) within 6 days to reduce severity.
What to do If your child gets measles?
Rest and hydration – Encourage fluids to prevent dehydration.
Fever management – Use acetaminophen (Tylenol) or ibuprofen (Advil) (avoid aspirin).
Vitamin A supplementation – Reduces measles complications.
Monitor for complications – Seek urgent medical care if the child has:
- Difficulty breathing
- Severe dehydration (not urinating, very weak)
- Seizures
Health officials strongly urge parents to vaccinate children to prevent further outbreaks. If your child shows any such symptoms described above, please contact the child’s pediatrician, or seek advice from your primary care.
References:
- https://www.cdc.gov/measles/signs-symptoms/index.html
- https://www.washingtonpost.com/health/2025/02/13/measles-outbreak-gaines-texas/
- https://fox59.com/news/national-world/measles-outbreak-sickens-dozens-of-kids-in-texas-whats-your-states-vaccination-rate/amp/
Image credit: Dave Haygarth https://www.flickr.com/photos/minnellium/ (https://flic.kr/p/6ixHyo) CC by 2.0 (Free for commercial use)
Author: Sumana Rao | Posted on: February 19, 2025
« Ways To Manage Twins Tantrums Noise Induced Hearing Loss In Children – Causes And Prevention »